ZELTA ECOA
Connect clinical trial participants, sites, and sponsors

OVERVIEW
Engage directly with clinical trial participants
Zelta’s electronic clinical outcomes assessment (eCOA) and electronic patient-reported outcomes (ePRO) technology enables clinical trial participants to submit reliable data with ease and convenience – entering diaries, surveys, and assessments wherever and whenever required. Trial managers can design patient-centric clinical trials that enhance the user experience, reduce administrative burden, and improve patient adherence.
-
eCOA/ePRO
-
eConsent
Patient-friendly approach
Make it convenient for patients to record data via computer, tablet, or smartphone.
Accessible and scalable
Leverage eCOA built directly into a cloud-based unified platform that is accessible via single sign-on for all of your trials.
Real-time results
Monitor participant adherence and reported results real-time.
Video
Fast tracking clinical trials with ePRO and self-service
Listen to Katrina Riggs, Chief Operating Officer for Fast-Track Drugs and Biologics, share how her team has leveraged Zelta's ePRO capabilities and self-service support to accelerate clinical trials with dozens of patient-reported outcomes and put their clients' data management needs and drug development processes on the fast track.

Deliver the best experiences for clinical trial participants and site personnel
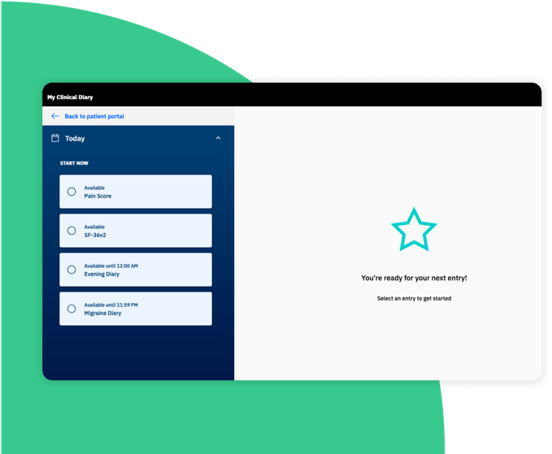
Zelta eCOA/ePRO: Boost patient engagement
-
Data gathered directly from participants, including diaries, surveys, and assessments, is instantly available in Zelta EDC.
-
Improve patient experience and adherence with an ePRO app that’s convenient and easy to use via mobile (iOS or Android) or web.
-
Reach participants and sites anywhere in the world.
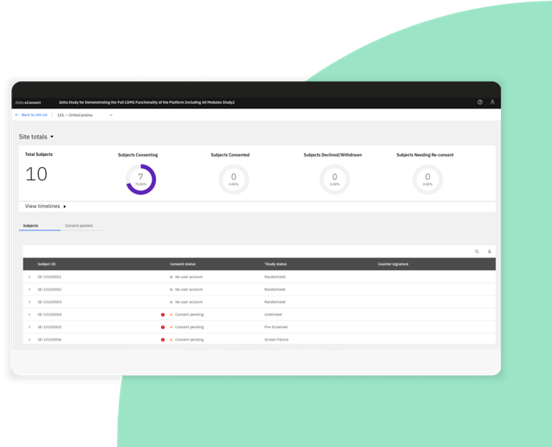
Zelta eConsent: Quickly consent participants wherever they are
-
Maximize site efficiency and remote management of participant consent.
-
Manage eConsent directly from Zelta’s cloud-based platform, alongside the eCOA/ePRO module.
-
Track amendments, consent status, and timelines in a dedicated dashboard.
-
Increase participant convenience with single sign-on access to both eConsent and eCOA/ePRO.
eCOA and ePRO unleash the benefits of decentralized trials
Decentralized clinical trials (DCTs) offer several benefits for CROs and sponsor companies, including reduced need for site travel, lowered trial costs, and better patient-reported outcome data for analysis.
Enabling trial participants to report outcomes via ePRO or using eConsent can greatly improve the patient experience, boost recruitment for trial managers, and allow real-time data capture. To date, the Zelta platform – including eCOA, ePRO, eConsent, and other modules – has been used to successfully build, run, and support over 250 hybrid trials or DCTs.
RESOURCES
Explore what Zelta has to offer
Fast tracking clinical trials with ePRO and self-service
Find out how Fast-Track Drugs and Biologics leveraged Zelta’s ePRO capabilities and self-service support to accelerate clinical trials with dozens of patient-reported outcomes.
How Zelta eCOA/ePRO captures data directly from clinical trial participants
Get all the technical details you need about the benefits of implementing eCOA/ePRO with Zelta.
Decentralized clinical trials are closer than you think
Read more about how DCTs give clinical trial developers the opportunity to create more patient-centric designs – better and faster than before.
