Clinical development
Get to market faster with a unified, cloud-based clinical data acquisition and management system.
Filters
Showing results for

Zelta user success stories: Less stress, lower database costs, and fast-tracked clinical trials
Read about how Zelta brings database builds in-house, fast tracks clinical trials...
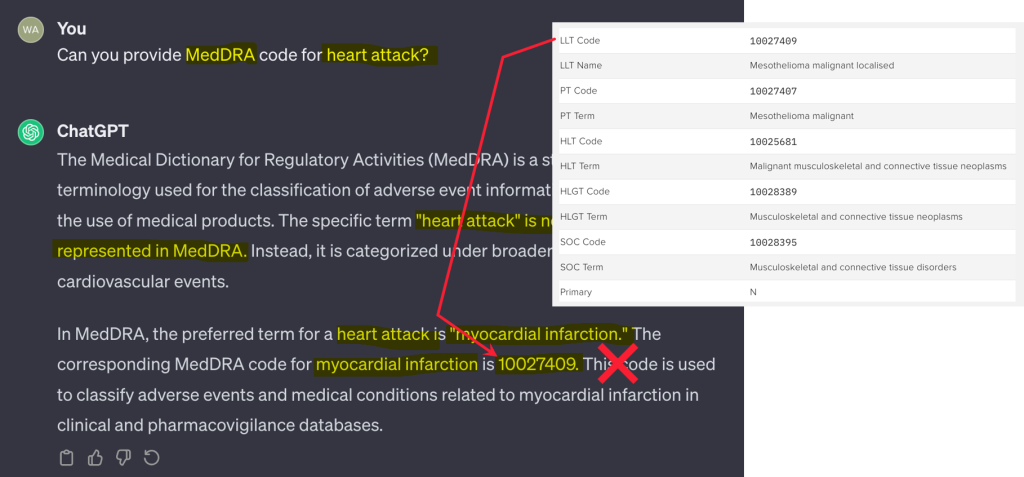
How supervised machine learning optimizes AI use in clinical trials
LLMs are frequently hailed as revolutionary tools in clinical trials. Read how...

Clinical trial trends in 2025: Investment headwinds, wearables, and targeted AI uses
This article originally appeared on Pharmaceutical Technology.2025 is set to be...

A toothy approach to innovating randomization in clinical trials
In a recent webinar, Solventum revealed how the Zelta eClinical platform helped...

The people-first approach transforming eClinical processes
Adding a “human at the center” perspective can help optimize clinical data...

The benefits of using the same clinical trials platform across phases
Sponsors can achieve greater efficiencies with a nimble clinical trials platform,...

How SaaS platforms are breaking the mold for clinical trials
With the right platform, SaaS systems can improve data accuracy, optimize costs, and...
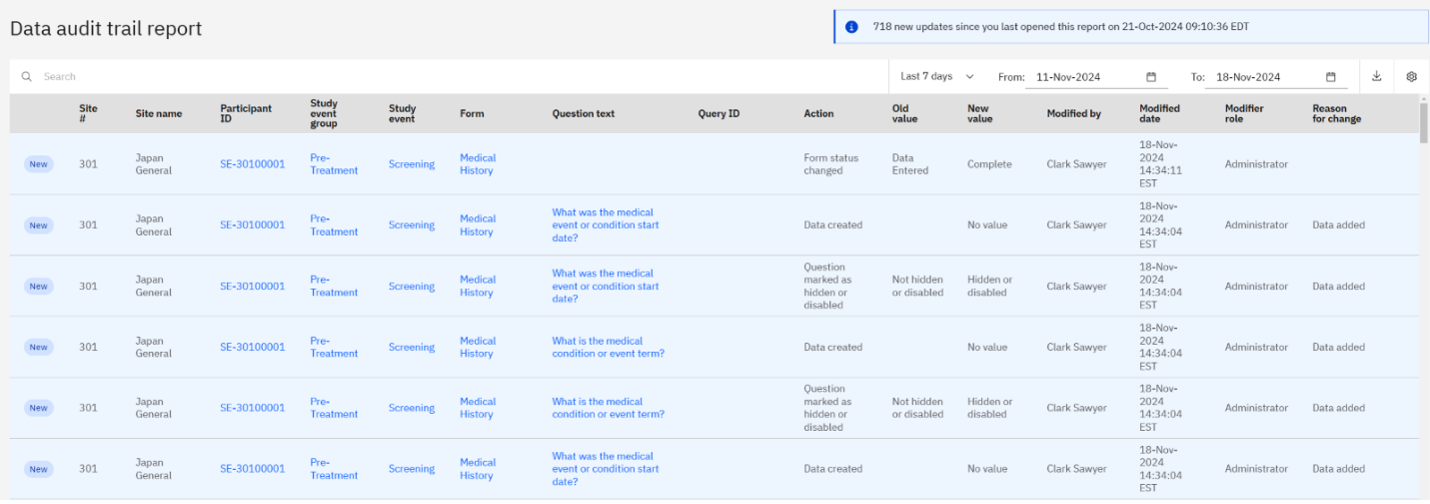
Zelta ranks as Major Contender in Everest Group’s 2024 Clinical Data and Analytics PEAK Matrix report
We’re excited to announce that Zelta has been positioned as a Major Contender in the...
Let the use case decide: The Zelta way on AI and supervised machine learning
For decades, when people thought of artificial intelligence (AI) what usually...

Zelta user success stories: Faster timelines, cleaner data, in-house flexibility
This month we’re profiling just a few of the CROs, sponsors, biotech, and pharma...

Zelta ranked as a Leader in Everest Group’s 2024 EDC Products PEAK® Matrix Assessment
We’re excited to announce that Zelta has been officially ranked as a Leader in the...

Gene therapy: How Veristat cut trial database costs by 30%
This piece originally appeared in Pharmaceutical Technology. The gene therapy...

Untapped opportunities: Fulfilling the promise of decentralized clinical trials
This piece originally appeared in Pharmaceutical Technology. The uptake of...

4 ways the vaccine rush is defining infectious disease trends for CROs in 2024
This piece originally appeared on Pharmaceutical Technology. The global healthcare...

The future of Zelta on display at the 2024 User Conference
That’s a wrap on the 2nd annual Zelta User Conference! We had a wonderful,...

Oncology in 2024: The clinical trial trends reshaping the role of CROs
This piece originally appeared on Pharmaceutical Technology. Oncology is the most...

Four ways a holistic ecosystem for clinical trials can help boost ROI
This piece originally appeared on Clinical Leader Clinical trials are complex and...

Zelta surpasses 4,000 clinical trial studies
February 2024 marked a major milestone for Zelta: the platform has now hosted over...

Can Your Clinical Trial Design Handle Mid-Study Changes?
As clinical research advances, the pharmaceutical and biotech industries...

Zelta expands its clinical trial ecosystem with a new partnership
At Zelta, we understand that collaboration is the name of the game when it comes to...

Lifesaving oncology research deserves cutting-edge solutions
The race to develop novel, advanced therapeutics for cancer patients is fast and...

New report confirms Zelta by Merative is a Major Contender
Merative has been named a Major Contender in Everest Group’s Life Sciences Clinical...

Merative’s first year: Focus and progress
Standing up Merative was a bold, complex move. To mark our first anniversary on 1...

Three years after COVID-19, the conversation around DCTs is shifting again
While interest in decentralized clinical trials (DCTs) has been heating up lately,...

The disconnect between clinical trial technology and legacy habits
In today’s age of cloud computing and data integration solutions, there are simple...

Merative Clinical Development has a new name
General Manager Jennifer Duff describes what the new name means for Merative’s...

Connecting with the clinical development community
The time it takes to design, launch and complete clinical trials is a problem many...

Will DCTs finally democratize clinical trials?
Zelta Product Leader Walker Bradham and Senior Product Manager Wes Fishburne...

COVID-19 pandemic and patient centricity in clinical trials
Jennifer’s career as a clinical data management and clinical development executive...

Decentralized clinical trials are closer than you think
Jennifer’s career as a clinical data management and clinical development executive...